ISSUE1741
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Michael Viscusi, Pharm.D., Associate Editor has disclosed no relevant financial relationships.
- Discuss the new monitoring requirements for lecanemab (Leqembi) and the new subcutaneous formulation of the drug (Leqembi Iqlik) for treatment of Alzheimer's disease.
The FDA has recommended additional MRI monitoring during initiation of treatment with the amyloid beta-directed monoclonal antibody lecanemab-irmb (Leqembi)1 and has approved a new subcutaneously injected formulation of the drug (Leqembi Iqlik) for maintenance treatment of Alzheimer's disease.
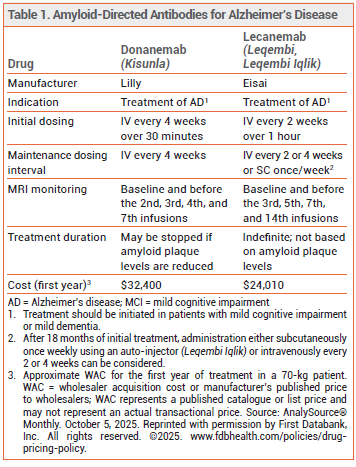
Additional MRI Monitoring – A Drug Safety Communication from the FDA recommends that patients undergo one additional MRI scan during initiation of lecanemab therapy to identify amyloid-related imaging abnormalities with edema (ARIA-E) earlier. The label for lecanemab will now recommend a brain MRI scan before the third IV infusion of the drug in addition to the previously recommended scans at baseline and before the fifth, seventh, and fourteenth infusions.2
According to the Drug Safety Communication, a total of 101 cases of serious ARIA-E associated with lecanemab, including 6 fatal cases, were reported to the FDA Adverse Event Reporting System (FAERS). Two cases occurred between the second and third infusions of lecanemab, and 22 cases (4 fatal) occurred between the third and fourth infusions. All 24 of these early cases were identified through unscheduled MRI scans that were performed because symptoms of ARIA-E developed.2
The same number of brain MRI scans are now required for lecanemab and donanemab (Kisunla), another amyloid beta-directed monoclonal antibody approved for treatment of Alzheimer's disease (see Table 1).3,4 Testing for APOE ε4 status should also be performed before starting either drug; the risk of ARIA is greater in patients who are APOE ε4 allele carriers (especially APOE ε4 homozygotes).
Patients receiving lecanemab or donanemab and their caregivers should be instructed to immediately report symptoms of ARIA-E (e.g., headache, confusion, dizziness, vision changes, nausea, aphasia, weakness, seizure). An MRI scan should be performed promptly if such symptoms develop, and treatment should be suspended in patients with moderate or severe ARIA-E symptoms or radiologic findings.
A New Subcutaneous Formulation – Subcutaneous lecanemab (Leqembi Iqlik) is FDA-approved for maintenance treatment of Alzheimer's disease in patients who have received IV lecanemab for at least 18 months. Leqembi Iqlik is the first subcutaneously injected amyloid beta-directed antibody product to become available in the US.
FDA approval of SC lecanemab was based on the results of substudies of an open-label extension trial (CLARITY AD; available as a presentation). Patients who were switched from IV to SC lecanemab after 18 months of treatment had similar changes in dementia severity (measured using the Clinical Dementia Rating-Sum of Boxes [CDR-SB] scale) and amyloid protofibril concentrations through 48 months compared to those who continued receiving IV dosing. Switching to SC lecanemab has been generally well tolerated; systemic administration-related reactions have occurred in ≤1% of patients.5
The recommended initial dosage of lecanemab is 10 mg/kg infused intravenously over 1 hour every 2 weeks. After 18 months, patients may receive lecanemab 10 mg/kg intravenously every 2 or 4 weeks,6 or lecanemab 360 mg injected subcutaneously once weekly into the abdomen, thigh, or back of the upper arm. The SC injection can be administered at home by the patient or a caregiver. Leqembi Iqlik auto-injectors should be stored in a refrigerator; they should sit at room temperature for 20 minutes before use.
An FDA review of SC lecanemab for initial treatment of Alzheimer's disease is ongoing.7
- Lecanemab (Leqembi) granted full approval for early Alzheimer's disease. Med Lett Drugs Ther 2023; 65:129.
- FDA Drug Safety Communication. FDA to recommend additional, earlier MRI monitoring for patients with Alzheimer's disease taking Leqembi (lecanemab). August 28, 2025. Available at: https://bit.ly/47iqc1H. Accessed October 23, 2025.
- Donanemab (Kisunla) for Alzheimer's disease. Med Lett Drugs Ther 2024; 66:129.
- In brief: A new donanemab (Kisunla) dosing regimen for Alzheimer's disease. Med Lett Drugs Ther 2025; 67:134.
- MC Irizarry and L Reyderman. Lecanemab subcutaneous formulation for maintenance dosing: the potential of a new and convenient option for ongoing treatment in early Alzheimer's disease. Alzheimer’s Association International Conference (AAIC) 2025; Toronto, ON; July 27-31, 2025.
- In brief: Once-monthly lecanemab (Leqembi) for Alzheimer's disease. Med Lett Drugs Ther 2025; 67:64.
- Eisai. Eisai initiated rolling supplemental biologics license application to the U.S. FDA for Leqembi Iqlik (lecanemab-irmb) as a subcutaneous starting dose for the treatment of early Alzheimer's disease under fast track status. September 3, 2025. Available at: https://bit.ly/4782V16. Accessed October 23, 2025.