ISSUE1713
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Michael Viscusi, Pharm.D., Associate Editor has disclosed no relevant financial relationships.
- Review the efficacy and safety of inhaled epinephrine (neffy) for emergency treatment of type 1 hypersensitivity reactions including anaphylaxis.
- Description: The first epinephrine nasal spray.
- Indication: Emergency treatment of type 1 hypersensitivity reactions including anaphylaxis in patients who weigh ≥30 kg.
- Clinical Studies: In studies in healthy subjects, the nasal spray appeared to be pharmacologically comparable to injectable epinephrine.
- Adverse Effects: Throat irritation, headache, nasal discomfort, a jittery sensation, tremor, and rhinorrhea can occur.
- Dosage: One 2-mg spray intranasally; the dose can be repeated 5 minutes later if symptoms do not improve.
- Cost: The wholesale acquisition cost of a package containing two single-use devices is $710; according to the manufacturer, the cost for most patients with commercial insurance will not exceed $25.
- Conclusion: The neffy nasal spray could be more convenient to use than injectable epinephrine for some individuals. It has not been studied in persons with underlying structural nasal conditions.
Outline
Table |
The FDA has approved an epinephrine nasal spray (neffy – ARS Pharma) for emergency treatment of type 1 hypersensitivity reactions including anaphylaxis in patients who weigh ≥30 kg. It is the first noninjectable epinephrine product to be approved for this indication.

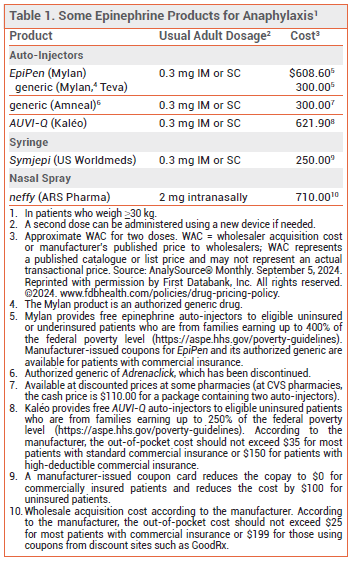
INJECTABLE EPINEPHRINE — Multiple epinephrine auto-injector formulations are available for emergency treatment of anaphylaxis (see Table 1). EpiPen and its generics have been used effectively for years. A generic version of Adrenaclick (brand no longer manufactured) is similar to EpiPen in size and functionality. AUVI-Q provides visual signals and audio instructions, has an automatic needle retraction system, and appears to be more convenient to carry and easier to use than EpiPen.1 Because of differences in device design and instructions for use, these auto-injectors are not interchangeable and pharmacists cannot substitute one for another.
An epinephrine prefilled syringe (Symjepi) is also FDA-approved for emergency treatment of anaphylaxis. It requires the user to manually inject the needle and push down the plunger, which may be difficult for some patients, particularly children.2
Some injectable epinephrine products have not been consistently available in recent years; at press time, EpiPen, the generic version of Adrenaclick, and Symjepi were on back order with no estimated return date.3
THE NEW FORMULATION — Each neffy nasal spray device contains a single 2-mg dose of epinephrine and is about the size of a teabag; the product is supplied in packages containing two devices. The drug delivery system is the same as that used in several other FDA-approved nasal sprays, including naloxone (Narcan). An absorption-enhancing agent (Intravail), which is also used in nasal spray formulations of drugs such as sumatriptan (Tosymra) and diazepam (Valtoco), increases epinephrine bioavailability.4
CLINICAL STUDIES — As with injectable epinephrine products for treatment of anaphylaxis, no efficacy trials were required for FDA approval of neffy. Approval was based on the results of five pharmacologic studies (summarized in the package insert) in a total of 175 healthy adults and 42 healthy children 8-17 years old who weighed ≥30 kg. Exposure to epinephrine and changes in blood pressure and pulse rate following a 2-mg dose of the nasal spray were comparable to those following a 0.3-mg IM dose, including in subjects with seasonal allergic rhinitis who underwent a nasal allergen challenge. The nasal spray has not been studied in persons with underlying structural nasal conditions, such as polyps or a history of nasal injury or surgery.
ADVERSE EFFECTS — The most common adverse effects of two doses of neffy in adults (incidence 7-19%) were throat irritation, headache, nasal discomfort, a jittery sensation, tremor, and rhinorrhea. Similar adverse effects were observed in children. The nasal spray solution contains sodium metabisulfite, which could cause a hypersensitivity reaction in patients with a sulfite allergy, but a history of sulfite sensitivity should not deter emergency use of the product.
DOSAGE, ADMINISTRATION, AND COST — The recommended dosage of neffy is one 2-mg spray administered into one nostril. If symptoms do not improve after 5 minutes, a second spray may be administered into the same nostril using a new device. The devices should be stored at room temperature, but excursions up to 50° C (122° F) are permitted; injectable epinephrine formulations only permit excursions to 30° C (86° F). At temperatures below -15° C (5° F), the nasal spray solution freezes and the device does not deliver epinephrine. Like other epinephrine products, neffy should be replaced before its expiration date. The shelf life of neffy is 30 months, which is longer than injectable epinephrine products (generally 12-18 months).
According to the manufacturer, the out-of-pocket cost for two neffy nasal spray devices should not exceed $25 for most patients with commercial insurance or $199 for those using coupons from discount sites such as GoodRx.5
CONCLUSION — The neffy nasal spray offers a new, potentially more convenient route of epinephrine delivery for emergency treatment of anaphylaxis in adults and children who weigh ≥30 kg. Whether its effectiveness differs from that of injectable epinephrine remains to be determined. Until data on the efficacy of neffy become available, some expert clinicians are advising patients to also carry an injectable epinephrine product. Neffy has not been studied in patients with underlying structural nasal conditions, such as polyps or a history of injury or surgery.
- Auvi-Q epinephrine auto-injector returns. Med Lett Drugs Ther 2017; 59:33.
- An epinephrine prefilled syringe (Symjepi) for anaphylaxis. Med Lett Drugs Ther 2019; 61:25.
- American Society of Health-System Pharmacists. Current drug shortages. Available at: https://bit.ly/3Qs7ETT. Accessed September 26, 2024.
- AK Ellis et al. Development of neffy, an epinephrine nasal spray, for severe allergic reactions. Pharmaceutics 2024; 16:811. doi:10.3390/pharmaceutics16060811
- ARS Pharma Press Release. ARS Pharmaceuticals receives FDA approval of neffy (epinephrine nasal spray), the first and only needle-free treatment for type I allergic reactions, including anaphylaxis. August 9, 2024. Available at: https://bit.ly/4cyySk4. Accessed September 26, 2024.