ONLY
ARTICLE
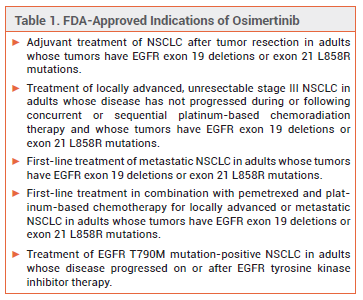
The oral kinase inhibitor osimertinib (Tagrisso – AstraZeneca), which has been available for years for treatment of non-small cell lung cancer (NSCLC) in adults with epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 L858R mutations, has now been approved for treatment of unresectable stage III EGFR-mutated NSCLC.1 About 20-30% of patients with NSCLC have locally advanced stage III NSCLC, and 60-90% of these patients have unresectable disease. Osimertinib is the first targeted therapy to be approved for the new indication.
MECHANISM OF ACTION — Osimertinib is a kinase inhibitor of EGFR; it binds to certain mutant forms of EGFR and disrupts EGFR signaling functions.
CLINICAL STUDIES — FDA approval of osimertinib for the new indication was based on the results of a double-blind trial (LAURA) in 216 patients with unresectable EGFR-mutated stage III NSCLC who did not have disease progression during or after chemoradiotherapy. Patients were randomized to receive osimertinib or placebo once daily until disease progression or unacceptable toxicity occurred. Median progression-free survival, the primary endpoint, was significantly longer with osimertinib than with placebo (39.1 months vs 5.6 months). About 65% of patients in the osimertinib arm were alive and progression free at 24 months compared to 13% of those in the placebo arm. Osimertinib reduced the risk of disease progression and the incidence of distant metastases, including new brain and lung lesions, compared to placebo. The objective response rate was higher (57% vs 33%) and the median duration of response was longer (36.9 months vs 6.5 months) with osimertinib than with placebo.2
ADVERSE EFFECTS — In the LAURA trial, the most common adverse effects of osimertinib were radiation pneumonitis, diarrhea, and rash. QT-interval prolongation and torsades de pointes, cardiomyopathy, keratitis, Stevens-Johnson syndrome, erythema multiforme major, cutaneous vasculitis, and aplastic anemia have been reported with use of the drug.
DRUG INTERACTIONS — Coadministration of CYP3A4 inducers3 can decrease serum concentrations of osimertinib and possibly its efficacy and should be avoided; if concurrent administration is necessary, the dosage of osimertinib should be increased to 160 mg once daily. Osimertinib can increase the exposure of drugs that are substrates of breast cancer resistant protein (BCRP) or P-glycoprotein (P-gp). Administration of other drugs that also prolong the QT interval can increase the risk of torsades de pointes.
DOSAGE, ADMINISTRATION, AND COST — Tagrisso is supplied in 40- and 80-mg tablets. The recommended dosage for the new indication is 80 mg taken once daily until disease progression or unacceptable toxicity occurs. The label contains dosage adjustments that should be made if adverse effects occur. Osimertinib should be discontinued if interstitial lung disease/pneumonitis occurs. The wholesale acquisition cost (WAC) of a 30-day supply of Tagrisso is $17,117.30.4
CONCLUSION — In one clinical trial, the oral kinase inhibitor osimertinib (Tagrisso) significantly extended progression-free survival and reduced the risk of disease progression in patients with unresectable stage III EGFR-mutated non-small cell lung cancer (NSCLC).
- Osimertinib (Tagrisso) for adjuvant treatment of NSCLC. Med Lett Drugs Ther 2023; 65:e131.
- S Lu et al. Osimertinib after chemoradiotherapy in stage III EGFR-mutated NSCLC. N Engl J Med 2024; 391:585. doi:10.1056/nejmoa2402614
- Inhibitors and inducers of CYP enzymes, P-glycoprotein, and other transporters. Med Lett Drugs Ther 2023 January 25 (epub). Available at: www.medicalletter.org/downloads/CYP_PGP_Tables.pdf.
- Approximate WAC. WAC = wholesaler acquisition cost or manufacturer’s published price to wholesalers; WAC represents a published catalogue or list price and may not represent an actual transactional price. Source: AnalySource® Monthly. October 5, 2024. Reprinted with permission by First Databank, Inc. All rights reserved. ©2024. www.fdbhealth.com/policies/drug-pricing-policy.