ONLY
ARTICLE
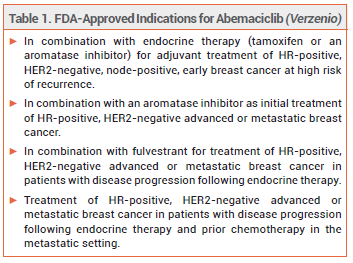
The oral cyclin-dependent kinase (CDK) 4/6 inhibitor abemaciclib (Verzenio – Lilly) has been approved by the FDA for use in combination with endocrine therapy (tamoxifen or an aromatase inhibitor) for adjuvant treatment of patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, node-positive, early breast cancer at high risk of recurrence.1 It was previously approved for the same indication, but patients were also required to have a Ki-67 score ≥20%. About 70% of all breast cancers are HR-positive and HER2-negative. Ki-67 is a prognostic biomarker for tumor proliferation; a score ≥20% is associated with early recurrence and poor prognosis.2,3
MECHANISM OF ACTION — CDKs 4 and 6 regulate the G1/S phase transition within the cell cycle; they are often overexpressed in HR-positive breast cancer, leading to cell cycle progression and cell proliferation. Inhibition of CDK 4/6 results in cell cycle arrest, senescence, and apoptosis.
CLINICAL STUDIES — FDA approval of abemaciclib for the new indication was based on the results of an open-label trial (monarchE) in 5637 women and men with HR-positive, HER2-negative, node-positive, resected, early breast cancer at high risk of recurrence (≥4 positive pathologic axillary lymph nodes or 1-3 positive axillary lymph nodes and at least one of the following: tumor size ≥5 cm, histologic grade 3, or Ki-67 score ≥20%). Patients were randomized to receive abemaciclib 150 mg twice daily for 2 years plus investigator-selected endocrine therapy or endocrine therapy alone for up to 10 years. At a median of 42 months, median invasive disease-free survival (IDFS) was not reached in either group. At 4 years, IDFS with abemaciclib plus endocrine therapy was 85.8% compared to 79.4% with endocrine therapy alone.4
ADVERSE EFFECTS — Abemaciclib can cause diarrhea, neutropenia, fatigue, leukopenia, nausea, anemia, and headache. In the monarchE trial, there were two treatment-related deaths in the abemaciclib plus endocrine therapy group and none in the endocrine therapy alone group.
DOSAGE, ADMINISTRATION, AND COST — The recommended starting dosage of abemaciclib for the new indication is 150 mg taken twice daily in combination with tamoxifen or an aromatase inhibitor. The drug should be taken for a total of 2 years or until disease recurrence or unacceptable toxicity occurs. A 30-day supply of Verzenio costs $15,571.80.5
- In brief: Abemaciclib (Verzenio) for early breast cancer. Med Lett Drugs Ther 2021; 63:199.
- A Fischer Maranta et al. Do you know the Ki-67 index of your breast cancer patients? Knowledge of your institution's Ki-67 index distribution and its robustness is essential for decision-making in early breast cancer. Breast 2020; 51:120. doi:10.1016/j.breast.2020.03.005
- R Nishimura et al. Ki-67 as a prognostic marker according to breast cancer subtype and a predictor of recurrence time in primary breast cancer. Exp Ther Med 2010; 1:747. doi:10.3892/etm.2010.133
- SRD Johnston et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol 2023; 24:77. doi:10.1016/s1470-2045(22)00694-5
- Approximate WAC. WAC = wholesaler acquisition cost or manufacturer's published price to wholesalers; WAC represents a published catalogue or list price and may not represent an actual transactional price. Source: AnalySource® Monthly. March 5, 2023. Reprinted with permission by First Databank, Inc. All rights reserved. ©2023. www.fdbhealth.com/policies/drug-pricing-policy.
