ISSUE1668
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Brinda M. Shah, Pharm.D., Consulting Editor has disclosed no relevant financial relationships.
- Discuss the use of finerenone (Kerendia) in patients with chronic kidney disease associated with type 2 diabetes.
Recently published guidelines from the American Diabetes Association (ADA) and the Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group recommend addition of the oral nonsteroidal mineralocorticoid receptor antagonist (MRA) finerenone (Kerendia) to standard treatment in patients with type 2 diabetes and chronic kidney disease (CKD).1,2
FINERENONE — Finerenone was approved by the FDA in 2021 to reduce the risk of sustained eGFR decline, end-stage kidney disease, nonfatal myocardial infarction (MI), hospitalization for heart failure (HF), and cardiovascular death in adults with CKD associated with type 2 diabetes.3 It inhibits aldosterone at the mineralocorticoid receptor, preventing receptor overactivation and decreasing the inflammation and fibrosis that lead to kidney dysfunction and cardiovascular disease. Like the steroidal MRAs spironolactone (Aldactone, and others) and eplerenone (Inspra, and generics), finerenone reduces albuminuria, but it appears to cause less hyperkalemia.
STANDARD TREATMENT — Patients with CKD associated with type 2 diabetes are usually treated with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB), in addition to a statin and metformin. A sodium-glucose cotransporter 2 (SGLT2) inhibitor is often added to slow progression of CKD and reduce the risk of cardiovascular events (see Table 1).1,2
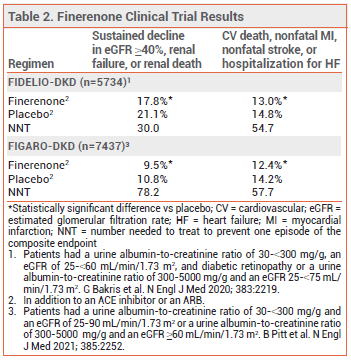
CLINICAL STUDIES — The recent recommendations were based on the results of two double-blind trials (FIDELIO-DKD and FIGARO-DKD) in patients with CKD and type 2 diabetes with or without cardiovascular disease. In both trials, patients were randomized to receive finerenone or placebo once daily in addition to a maximally tolerated dose of an ACE inhibitor or ARB. Most of the patients in FIDELIO-DKD had severely elevated albuminuria; in FIGARO-DKD, they had less severe albuminuria. In both studies, few patients were taking an SGLT2 inhibitor. Over median follow-up periods of 2.6 years (FIDELIO-DKD) and 3.4 years (FIGARO-DKD), the incidence of a composite of sustained decline in eGFR ≥40%, renal failure, or renal death and the incidence of a composite of cardiovascular death, nonfatal MI, nonfatal stroke, or hospitalization for HF were statistically significantly lower with finerenone than with placebo (see Table 2).4-6
ADVERSE EFFECTS — Hyperkalemia was the most common adverse effect of finerenone in FIDELIO-DKD and FIGARO-DKD. Hypotension and hyponatremia also occurred.
DOSAGE, ADMINISTRATION, AND COST — The recommended starting dosage of finerenone is 10 mg (eGFR ≥25-<60 mL/min/1.73 m2) or 20 mg (eGFR ≥60 mL/min/1.73 m2) once daily; after 4 weeks, the 10-mg dose can be increased to 20 mg. Finerenone should not be started in patients with a serum potassium level >5.0 mEq/L. Serum potassium levels should be monitored during treatment; the drug should be withheld when levels are >5.5 mEq/L. Finerenone is not recommended for use in patients with an eGFR <25 mL/min/1.73 m2 or in those with severe hepatic impairment (Child-Pugh C). A 30-day supply of Kerendia costs $597.60.7
CONCLUSION — Addition of the oral nonsteroidal mineralocorticoid receptor antagonist (MRA) finerenone (Kerendia) to standard treatment is now recommended to slow progression of kidney disease and reduce the risk of cardiovascular events in patients with type 2 diabetes and chronic kidney disease (CKD). Finerenone has been shown to modestly improve renal and cardiovascular outcomes compared to placebo in patients with type 2 diabetes and CKD, most of whom were not taking an SGLT2 inhibitor concomitantly. Finerenone is expensive and causes hyperkalemia. How steroidal MRAs such as spironolactone and eplerenone compare to finerenone in improving renal and cardiovascular outcomes in patients with type 2 diabetes and CKD remains to be determined.
- American Diabetes Association Professional Practice Committee. 11. Chronic kidney disease and risk management: standards of medical care in diabetes—2022. Diabetes Care 2022; 45(Suppl 1):S175. doi:10.2337/dc22-s011
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 clinical practice guidelines for diabetes management in chronic kidney disease. Kidney Int 2022; 102(5S):S1. doi:10.1016/j.kint.2022.06.008
- Finerenone (Kerendia) for chronic kidney disease. Med Lett Drugs Ther 2021; 63:131.
- GL Bakris et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020; 383:2219. doi:10.1056/nejmoa2025845
- G Filippatos et al. Finerenone and cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes. Circulation 2021; 143:540. doi:10.1161/circulationaha.120.051898
- B Pitt et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021; 385:2252. doi:10.1056/nejmoa2110956
- Approximate WAC. WAC = wholesaler acquisition cost or manufacturer's published price to wholesalers; WAC represents a published catalogue or list price and may not represent an actual transactional price. Source: AnalySource® Monthly. January 5, 2023. Reprinted with permission by First Databank, Inc. All rights reserved. ©2023. www.fdbhealth.com/policies/drug-pricing-policy.