ISSUE1364
The FDA has approved the marketing of fentanyl sublingual tablets (Abstral – ProStrakan) for treatment of breakthrough pain in adult cancer patients who are already receiving and are tolerant to opioid therapy. It is the fourth transmucosal formulation of fentanyl to become available in the US for this indication.1-3
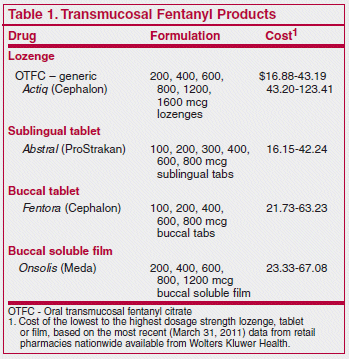
The manufacturer recommends an initial dose of 100 mcg, a maximum of 2 doses per breakthrough pain episode, and use for no more than 4 breakthrough pain episodes per day. As with all formulations of fentanyl, strong inhibitors of CYP3A4 such as clarithromycin (Biaxin, and others) or itraconazole (Sporanox, and others) can increase serum concentrations of the drug to levels that can cause respiratory depression even in opioid-tolerant patients. A single dose of any formulation of transmucosal fentanyl could be fatal for a child.
